Following up from the last post…
(A lil’ later than one week, but who’s counting)
Today, we’ll do a deep dive into Li-ion batteries given their importance, and we’ll cover lithium-metal and solid-state in the next post given their hype. In order to understand the latter two, you’ll need to first understand Li-ion, so get ready. Battery electrification is like going through puberty—lots of uncertainties, dizzying growth spurts (though at 5’5” I’m still waiting on mine), peer pressure, and tons of drama. The best way to keep your feet on the ground is to have a decent technical understanding of how things work. “Technical” ≠ hard, boring stuff. It just means common sense at the root level.
Today, we’ll deep dive in a way I guarantee you’ll understand. Long (as always) but I promise it’s worth your time, and there are a ton of images to look at :) We’ll cover Li-ion batteries like a kid with a scroll-wheel on Google Earth: by zooming in on the planet until we get to the blurred-out license plates in Street View. You are welcome to jump off at the end of each zoom level with increasing degrees of understanding (but I know ya wanna nerd out and soak it all in 🤓 ). By then, we’ll be able to breeze through lithium-metal and solid-state batteries in the next post without glazed eyes or heart palpitations. At the end of the next post, we’ll also discuss the roles these lithium-based batteries have to play in reshaping our energy demands, which is what brought us here in the first place.
Sound good? Great. We’re goin’ in.
Initiate the Zoom
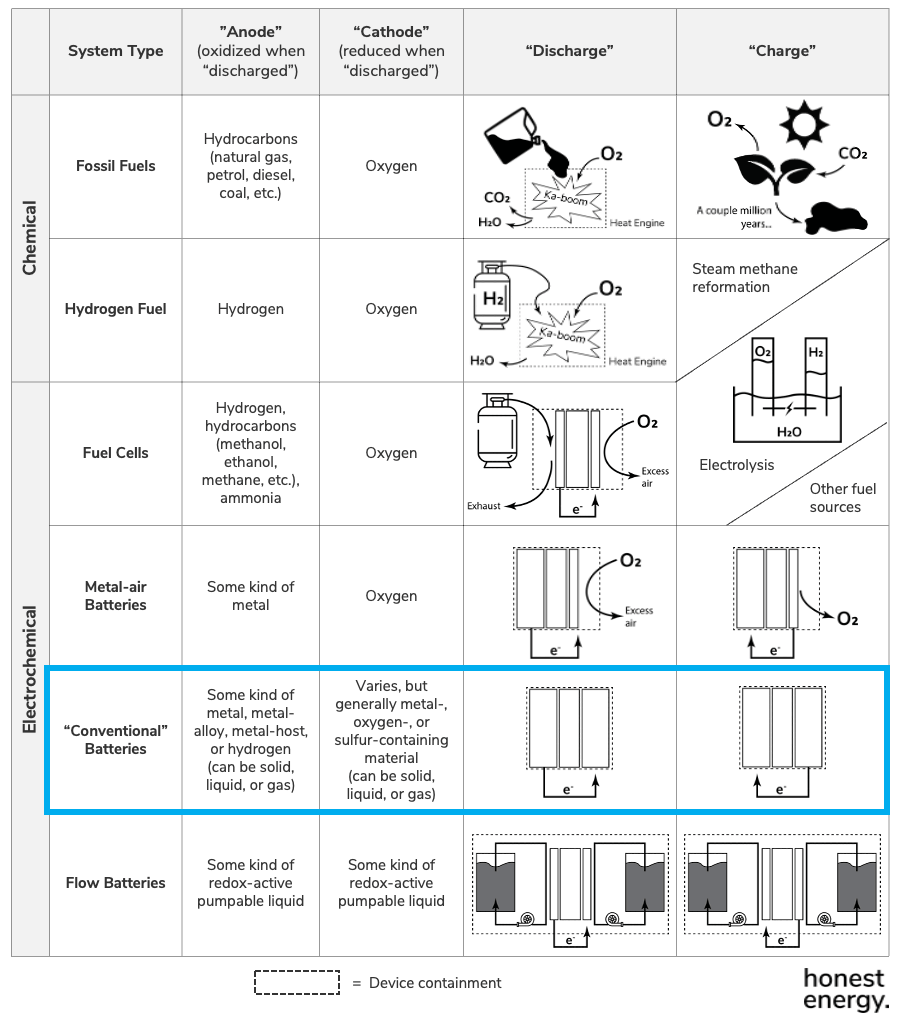
We start off with a global high-level view of batteries, and we established this last time by understanding what batteries really are from a first-principles perspective. If you haven’t read the last post, I recommend you do first.
As a reminder, here’s a recap of chemical and electrochemical storage systems. Li-ion, lithium-metal, and solid-state batteries all fall under the class of “conventional batteries”:
Our first zoom-in
Since Sony was the first to commercialize the Li-ion battery, we’ll honor them by zooming-in on Japan for this one. We’ll take our first zoom-in step, and look at things at the cell-level.
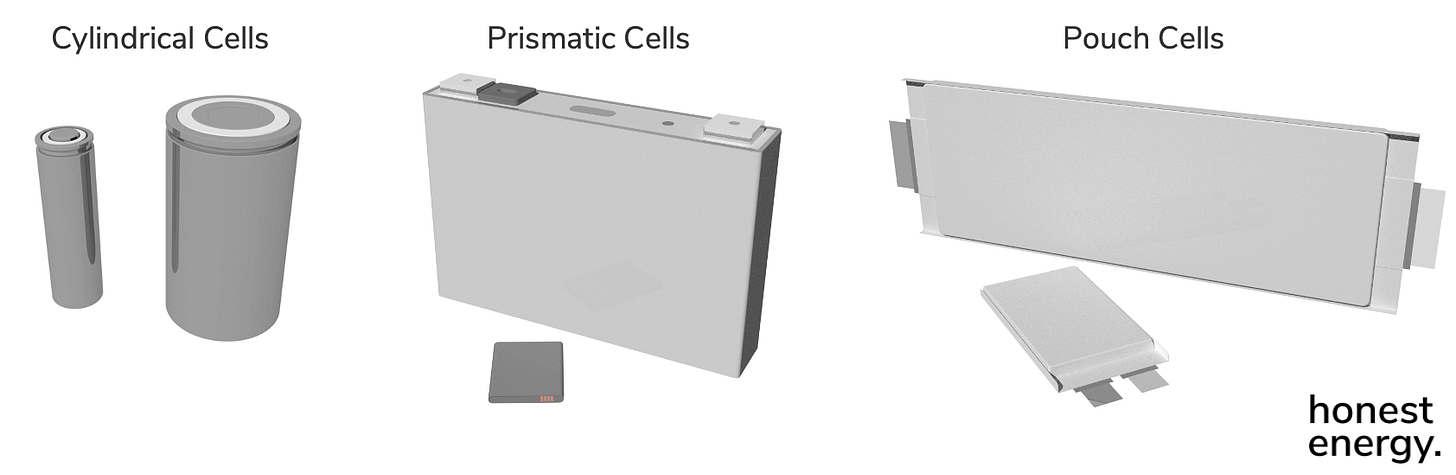
Batteries are made up of individual cells. In your phone, there is typically only one cell, and can be interchangeably called a battery. In your laptop, there are several. In your EV, there are hundreds or thousands. Cells packed together in a battery are known as…well, a pack. We won’t talk about battery packs today, but they’re pretty sexy too from an electrical, mechanical, and software perspective. Each cell is a self-contained, sealed package with its own electrochemical environment within. Cells can typically look like any of the three form factors:
Cylindrical and prismatic cells are different from pouch cells in that they have rigid casings, typically made from stainless steel or aluminum. Pouch cells, on the other hand, are enclosed using a material not unlike your vacuum sealed bag of coffee beans: a triple-layered laminated film with aluminum half the thickness of your hair (~40µm) sandwiched between two plastic layers. The reason we must have metals in our enclosures, including even the pouch material, is that they are better barriers to smaller molecules like oxygen that lowly-plastic bags cannot properly seal against. Since Li-ion cells are insular and antisocial with the outside world, not to mention the highly corrosive environment within, it’s important there is no exchange of materials in or out from each cell.
As you may have guessed, non-rigid pouch materials are much lighter than hard metallic cases, which helps increase the energy per unit weight (specific energy) of these cells. But since they are compliant and only have the pressure of the Earth’s atmosphere clamping down on the vacuum inside, they are more prone to dimensional changes with each charge/discharge cycle and throughout their service life (remember, batteries are moving parts). This means they may require additional clamping mechanisms that could end up eating away at specific energy gains. Since there’s only a thin pouch material enclosing the stuff inside, they can also sometimes be less safe. But as we talked about last time, the world of batteries (and engineering in general) is a world of tradeoffs, and so there’s certainly no “best” design.
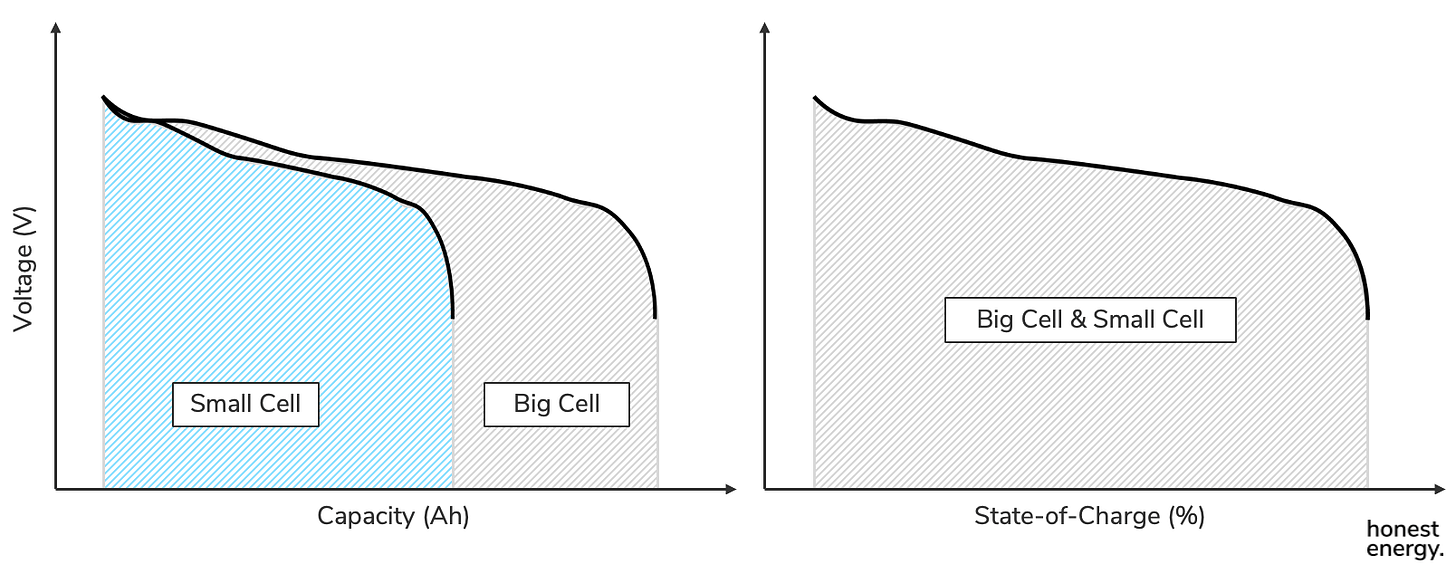
Of the above form factors, cells can also span a significant spectrum in size, ranging from tiny ones used in your airpods to gigantic ones used in some EVs. When sizes change, the capacity of the cells will change but their voltage will not, so long as the same types of Li-ion chemistries are used. That’s because the voltage is dictated by the various stages of electrochemical reactions, and they don’t change just because we made a bigger battery cell. We’ll talk about the different Li-ion chemistries later. Here’s a visual to help illustrate the point. Note that voltages are the same:
One key challenge in the Li-ion battery industry is that there exists almost no standardization in the cell form factors. The only ones that do exist are in the cylindrical space, which were originally legacy designs from older laptops and consumer electronics (e.g. the 18650 cell), but new ones are now being instantiated by market leaders (e.g. 2170s, 4680s). There is some effort now to standardize prismatic cells for EV applications, but pouch cells continue to exist in the wild. Each version of the iPhone (1 through…what number are we on now?) all used different sized pouch cells, which you can imagine to be kind of a nightmare from a supply perspective. Well…it’s still the case today.
Understanding V, Ah, A, Wh, W, SOC, OMG, LOL, C-rates, and efficiencies
Capacity is a measure of how much stuff something can hold—in this case charged ions and electrons, like water in a bucket or tub (note that this is a different use of “capacity” when talking about the electric grid, which refers to capacity as a power metric). And voltage, as you may recall from our post about the electric grid, is just the electrical analogy to altitude. The energy contained in a cell is just the amount of water in the bucket held at a given height, much like mechanical potential energy (PE = mgh). Because of this, the energy in the two examples below could have the same amount of energy.
Current is the rate at which the charged ions and electrons are flowing, but it doesn’t contain any information about the height (voltage). Power does however, and similarly, the two examples below could have the same amount of power output.
You may have noticed that current is measured in Amperes (cool kids call it amps), whereas capacity is measured in amp-hours. This makes sense because if you pour the water at a steady flow rate (amps) for a given time (hours), that gives you the amount of water (capacity; amp-hours) you “discharged”. Since current is a measure of the amount of moving charge per second, capacity is then just a measure of charge. In this case, it is representative of the actual number of lithium ions and/or electrons (they’re the same since one lithium ion is paired with one electron) that have been passed in either direction of charging/discharging. Apart from amp-hours, capacity can also be measured in Coulombs, but we use amp-hours because using the latter would have a lot more zeros attached (multiply by 3,600) and nobody likes that.
Since the voltage of a cell is dictated by the electrochemical reactions, they are predetermined values. In typical Li-ion cells, the max voltage is 4.2V, the min voltage 2.5V, and a nominal voltage of ~3.7V. But these are variable depending on cell chemistry, manufacturer, and what the cells are designed to do (again, we’ll talk about this later). But if you ever see a voltage > 5V in a commercial cell, either you’ve mistaken it for multiple cells connected in series, or go hide behind a wall because it’s about to blow. No cells on the market can charge to that high of voltages today (and likely won’t be anytime soon). In reality, the voltage curves on discharge look something like the following.
Note that energy is just the area under the curve (a.k.a. the integral) on a voltage vs. capacity graph. But since regular people can’t do integrals of arbitrary curves in their heads, the nominal voltage is a useful way to calculate energy by simply multiplying by capacity. If the exact same Li-ion chemistries (and electrode designs) are used in the big EV cell versus the tiny airpod cell, their voltage profiles stay the same but just scale by their capacities in the x-direction. If you normalized their capacities, which would now be called state-of-charge, the voltage curves would look identical, and you wouldn’t be able to tell one from the other just by looking at the voltage vs. SOC plot.
Finally, a note about efficiencies. You may have heard that Li-ion batteries are highly efficient devices, but you may also have come across numbers ranging from 85% to 99.995%. So which is it?? Now that we know what energy and capacity are, let’s look at the charge/discharge curves of a typical Li-ion cell.
Note that the charge voltage will always be higher than the discharge voltage. That’s because we live in an imperfect world with no free lunch, and more work needs to be put in than you can get out. And in between the charge and discharge voltage curves, we have the open circuit voltage (dashed) when no energy is being injected or extracted from the cell. This is the voltage the cell really wants to be at when unbothered by us humans, and the voltage will “bounce back” to this dotted line when you stop charging/discharging at any point…kinda like a spring.
If you try to increase the rate of charge or discharge (also known as C-rates), the voltages you measure will deviate further away from this happy, open circuit voltage. This is known as polarization, and we’ll talk more about it later. But just know that at any state-of-charge, a battery cell will have a true open circuit voltage it wants to be at—either higher or lower than what is measured when discharging or charging, respectively. At too high of discharge rates (high C-rates) though, you could cause your voltage to hit the lower cutoff limit prematurely, even though there is still capacity “to be had”, at which point battery control electronics (which will always be paired with Li-ion cells in any electronic device) step in and shut the party down. You may have experienced this when trying to open a power-intensive app like Snapchat (do people still use this??) when it’s freezing cold outside and your phone powers off. Yes, polarization is very much temperature dependent. Btw, a 1C-rate will discharge your cell in ~1 hour, a 2C-rate in ~1/2 hour, and 0.1C-rate in ~10 hours (it’s an inverse relationship). C-rates are only approximate numbers.
The red shaded area between the charge and discharge voltages is then just our energy inefficiency—it represents the extra work we put in to charge the cell that we couldn’t get back. Given what we just learned, this efficiency will be dependent on the rates of charge/discharge. Generally, at “moderate rates”, the energy efficiencies are 93-98%. But why do some people boast numbers like 99.995%? Those numbers refer to Coulombic efficiencies, which is only looking at the capacity you get back on discharge compared to that of charge, but tells you nothing about energy. These values naturally need to be very high for any rechargeable system, as the Coulombic inefficiencies (shown above in the inset) accumulated every cycle will result in your capacity fade. If a cell had 99% Coulombic efficiency at moderate cycling rates (which may sound impressive at first), it would last only 22 cycles before it hit end-of-life, typically defined as 80% of what it originally had at beginning-of-life (0.99^22 = 0.80). Yeah, that sucks. So don’t swoon if someone boasts a 99% Coulombic efficiency—a housefly lives longer than that.
Finally, numbers as low as 85% refer to battery packs comprised of hundreds or thousands of cells that also require additional thermal management and computer smarts to make things work. Again, no free lunch, and the energy used for those additional things sap energy efficiency away.
Ok, let’s zoom-in again
Now that we understand the higher level technicalities, let’s zoom-in on the city of Tokyo to understand the stack-level details.
Taking the cells we looked at earlier, let’s strip em’ down 😏 . When we remove the packaging from the cells in the various form factors discussed above, we find two general types of cell construction: wound or stacked. This refers to the construction of the electrode and separator components within the cell. Cylindrical cells are always wound. Prismatic or pouch cells can either be flat wound or stacked. Here’s some progressive zooming-in for ya:
There are advantages and disadvantages of either construction method (as always). Winding things is generally easier from a manufacturing perspective since it can be done much quicker and therefore assist in reducing costs. But the bending radii created by wrapping things creates all sorts of complications that aren’t helpful for cycle life. We won’t dive too deep here, but just know that the cell construction of the electrode-separator stack is not trivial. The fact that Tesla could inject the words “tabless design” into the mainstream dialogue after their Battery Day tells you that these design considerations have big implications. Also, that people are huge nerds and I love it.
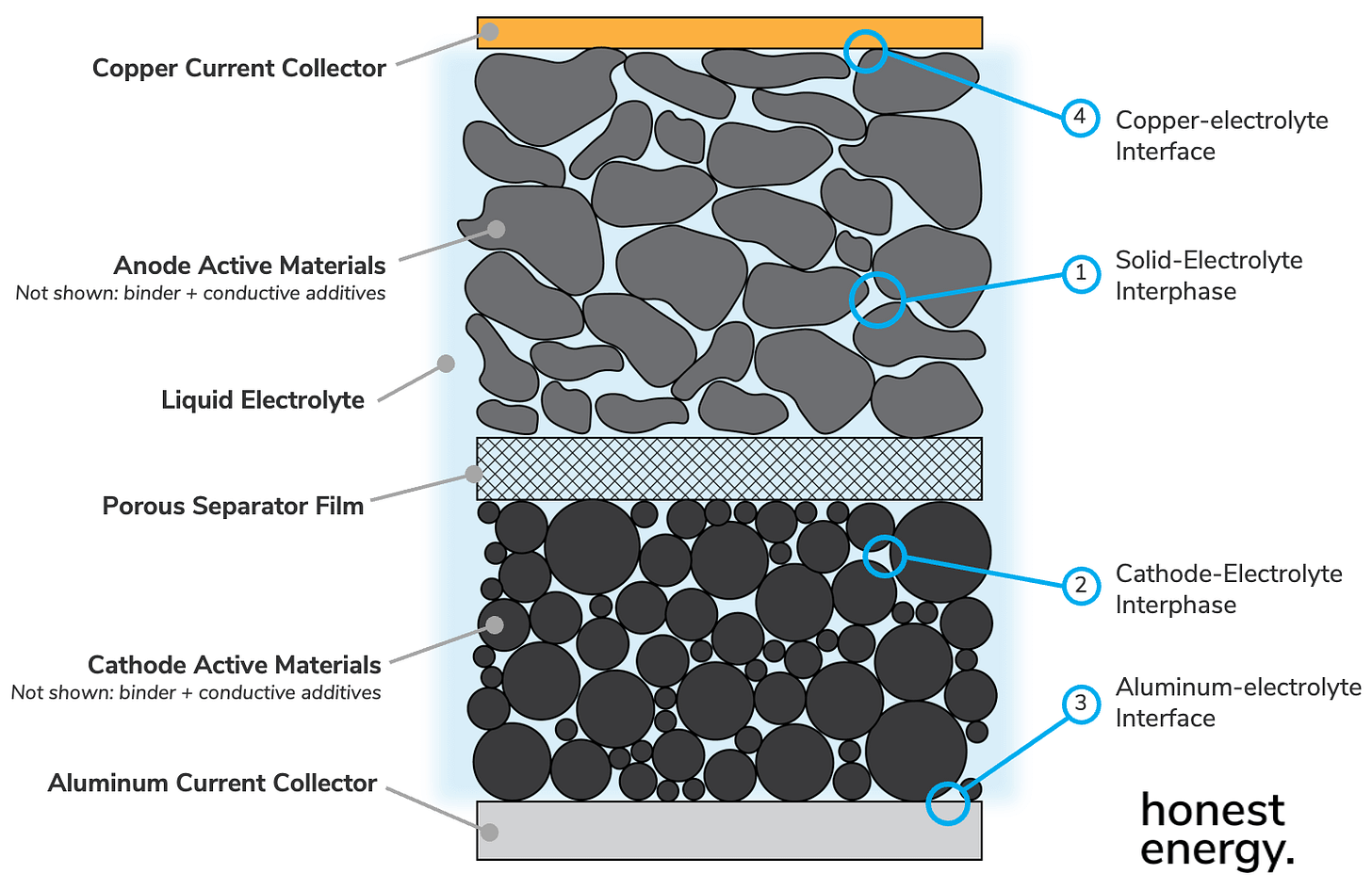
Regardless of cell construction though, you may notice that all share the same basic layered construction. We’ll zoom-in again on the following components:
The above is the basic cell stack found in every Li-ion cell. Note that the anode (the negative side) and cathode (the positive side) electrodes are coated on both sides of their respective current collectors (fancy term for metal foils)—copper for the anode and aluminum for the cathode. We’ll talk about why we use these metals shortly. Also note that the cathode electrodes are always sized smaller than anode electrodes and we’ll talk about this later too. These electrodes are separated by a creatively-named separator film, and everything in the cell is soaked in a liquid electrolyte.
For some reason, it has become common parlance in some circles (like hobby R.C. communities) to call the pouch cells we met earlier as “LiPo”—short for “lithium polymer”—where it is presumed that the electrolyte and separator are a polymer gel-like material instead of a liquid electrolyte. Well...this is wrong, so you should stop using it if you do. Pouch cells use the same super-liquidy electrolytes just like cylindrical or prismatic cells. The reason they don’t feel like water balloons is that Li-ion batteries really don’t need all that much electrolyte to function, and have just enough to fill the pores inside. We’ll talk about actual polymer electrolytes next time when we talk about solid-state, but they are certainly not in your drone today.
Again!
Technically, we can simplify this basic cell stack even more by zooming-in on the most elemental unit cell and understand things at the electrode-level. Here, we’re looking down at the Musashino neighborhood next to Tokyo from maybe ~10,000 ft.
Here’s the unit cell below zoomed-in. As you can see, both electrodes—the anode and the cathode—are porous coatings stuck onto the foils (current collectors). The electrodes are made up of mostly active material particles (usually >95% of the solid mass), with the remainder being: (1) conductive additives to help with electron transport from the active materials to the current collectors and (2) polymeric binders to glue everything together. These are sometimes known as “CBD”—no, not weed-juice but “carbon-binder distribution”. They’re not shown below, but they would be hard to see anyway.
The pores of the electrodes are critical for the cells to function since they are filled with electrolyte—the only means by which lithium ions can quickly travel long distances in the cell. This phenomenon is known as mass transport, and “quickly” is really not that quick when compared to electron transport. It’s actually quite sluggish, even though it’s in a liquid. Mass transport is one of the major limiters of power performance in today’s Li-ion cells (on charge and discharge), and depending on what we need, there are several design strategies to address it.
Electrode Thicknesses…duh!
If traveling long distances through pores is slow, then why not just reduce them by making electrodes thinner?
As stupidly simple as it sounds, electrode thicknesses are actually the primary tool battery engineers have to increase the power performance of cells (in any kind of battery, not just Li-ion). But of course, you should know by now that it’s a world of tradeoffs, so…what’s the catch? When in doubt, think energy density. The problem with making electrodes thinner is that you increase the overall number of layers of our unit cell to build up sufficient capacity to be useful. And since each unit of a unit cell requires an aluminum foil, a copper foil (heavy), and a separator film—all of which contribute no energy—they are dead weight and space hoggers.
So…for high energy density, we like electrodes nice and thicc, just like our batts 🍑 . What if instead, we just smooshed them more?
Smooshing
If we smooshed the crap out of our electrodes, couldn’t we make them thinner?
Yes, and this is a great strategy to pack the same amount of capacity into a thinner cell for increased energy density. But doing so will hurt mass transport even more because the lithium ions now have to travel through a difficult maze just to get to the other side. This is technically called tortuosity, and we don’t like it. So power performance will be garbage.
Like we said in the last post, it’s a world of tradeoffs, and what we just saw is the classic energy-power tradeoff. Li-ion batteries therefore span a wide range of designs—from super thin electrodes for mega-high discharge rates where needed, to super thick electrodes for maximum energy densities. But typically, a Li-ion cell cannot do both.
Shameless plug: there are some strategies to break this energy-power tradeoff, like what startup EnPower is doing. Full disclosure: I am the co-founder and CTO of EnPower 😬 . Opinions here are my own.
Getting technical for a hot sec
One common way to quantify electrode thicknesses is to define how much “stuff” there is on a unit area of foil—typically in terms of available capacity a square centimeter of cathode can provide. This is known as capacity loading in mAh/cm2, referring to only a single-sided coating like in our unit cell. It doesn’t have enough information for you to back-calculate actual electrode thicknesses without knowing other things, but battery engineers are weird and they use it as a proxy. Typical commercial Li-ion cells have capacity loadings ranging from 2.5-5.5 mAh/cm2, with the lower and higher values correlating to power- and energy-dense designs, respectively. The reason this is important to know (and for you to sound smart) is that a lot of published “battery breakthroughs” refer to research work done at very low capacity loadings (cool kids sometimes just use loadings) that mask a lot of the hard things in practical cells, among many others. We’ll go through some examples next time when we talk about solid-state. In your electric vehicle, loadings are almost always above 4.0 mAh/cm2 in order to provide practical range. Don’t believe any breakthroughs that predict incredible EV range if they are < 3 mAh/cm2.
What happens when we charge and discharge?
This is what charging and discharging look like.
During charging, four things happen simultaneously:
Lithium ions are evicted out of the cathode active materials and spat into the liquid electrolyte as they begin to make their way to the anode through the porous separator.
An equal number of electrons are ejected from the same cathode particles that delithiated (fancy term for losing lithium ions) to maintain charge neutrality. They travel through the CBD network to the current collector, pushing current out through an external circuit.
Random lithium ions that just happen to be hanging out in the pores of the anode electrode get absorbed into anode active materials on the other side.
An equal number of random electrons found in the CBD of the anode are pulled into the same anode active materials that lithiated to maintain charge neutrality.
There is Balance (in the Force). The exact opposite happens during discharge.
As you can see, if we wanted to charge our cell by exactly one lithium ion, it would be a misconception to think that the same lithium ion that got ejected from a cathode particle would be the same one that gets absorbed into an anode particle on the other side. Electrochemistry is impatient, and lithium ions and electrons are completely fungible (that’s an economic term for y’all), unlike NFTs. That just means they are hot-swappable, and the Li-ion cell doesn’t care which ions or electrons get used. Of course, we usually charge our cells by a tad more than one single ion (on the order of 10,000,000,000,000,000,000,000 ions), so there will be a net flux of ions traveling through the pores (as drawn) and a net flux of electrons in the external circuit.
And again…
Now let’s do our second-to-last level of zooming-in where we’re hanging out just above the roofs and streets. Here, we’ll start understanding some of the key interfaces in a Li-ion cell. A lot of what determines if a battery cell will even work, or just give you a dead flat line, often depends on key interfaces being formed correctly so this stuff is important. This is also the level-of-zoom where some of the trickiest challenges in solid-state batteries are found, which we’ll cover next time.
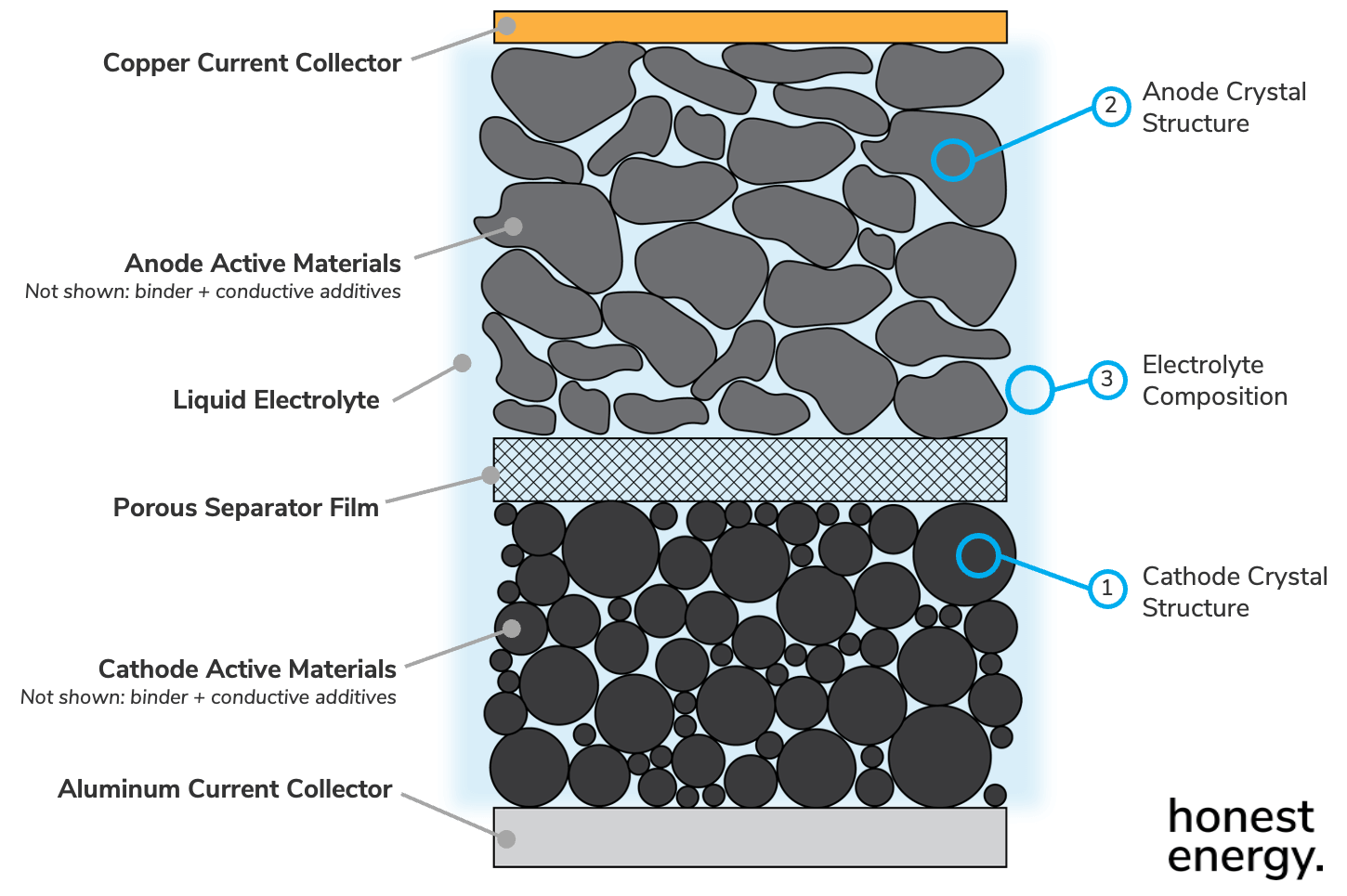
Today, we’ll zoom in on four interfaces circled below that are of key importance, one-by-one:
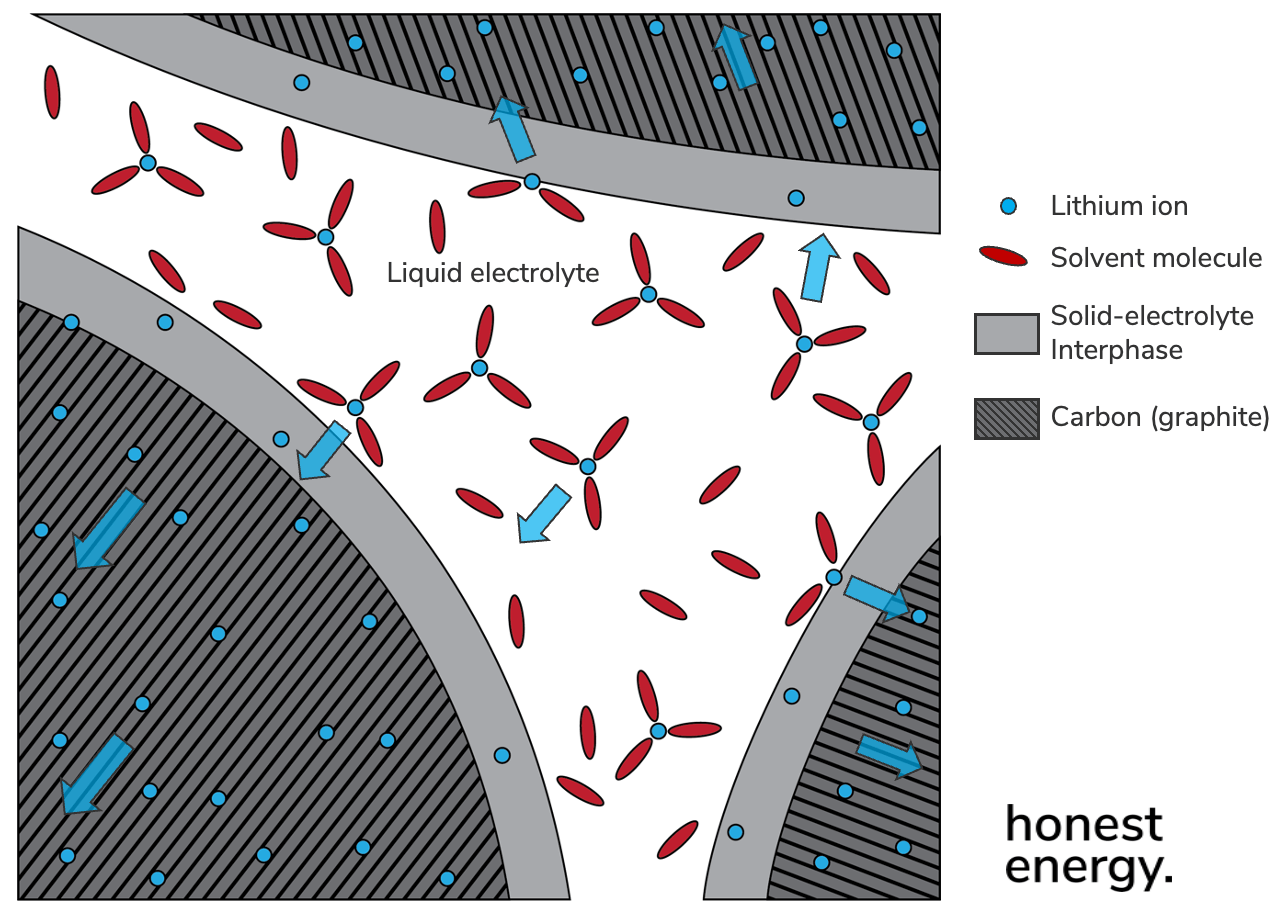
1. Solid-Electrolyte Interphase (SEI)
This is arguably the most important interface you need to know about, and yes you’re not the only one confused why it’s called an “interphase” as opposed to an “interface”. Regardless, it’s important to know that the components in a Li-ion battery are not entirely stable and happy just being together—they very much aren’t. Of special importance is the anode active materials.
Recall from last time how Li-ion batteries came to be, where our pal Akira Yoshino swapped pure lithium with a carbon host material to make things work. This solved the dendrite problem since we were no longer depositing lithium in its metallic form; instead we were just sticking it inside the carbon host material. Voilà. But who says the carbon was happy with this whole sitch?? It very much isn’t. Lithium is very reactive and has the lowest standard potential of any material—you can think of it like a bad attitude. When you stick lithium into other materials like carbon, they also develop a bad attitude and become reactive with their surroundings—which in the case of Li-ion would be the liquid electrolyte—and they undergo chemical side reactions that we don’t like, forming certain reaction byproducts.
As you know from middle school science, we have three states of matter, and the reaction byproducts can either be a gas, liquid, or solid:
If the reaction byproducts were a gas, they would just bubble away (causing your cell to balloon if it were a pouch cell), allowing more liquid electrolyte to react with the anode material surface, and continue doing so until there was no electrolyte left. Here, no bueno—the cell will never charge or function.
If instead the reaction byproducts were a liquid, they would only float away and similarly allow the reaction to continue until there was nothing left to react. The cell won’t balloon, but still, no bueno.
Only in the case of a solid reaction byproduct that happens to stay stuck onto the surface of the anode material particle is there any chance of success. But not only must the solid byproducts stay stuck, they must also form a completely insulating blanket around the entire particle to stop it from continuing to react with the liquid electrolyte, so called “self-passivating”. This is done on the first couple cycles of a Li-ion cell’s life, and if done properly, should not continue forming afterwards. It’s kind of like giving a cold, grumpy child a blanket and a hot chocolate to stop making a fuss. Finally, not only does the blanket layer need to be completely insulating to electrons so no reactions can continue (remember, chemical reactions are the social network of electrons), it still needs to be permeable by ions so the carbon active material host can actually absorb lithium to store any useful capacity.
So this is what zooming in on the SEI looks like on the surface of some carbon anode materials. Note that this is not to scale. The SEI is typically several tens of nanometers thick, or close to 300x the diameter of each lithium ion. The active materials, on the other hand, are typically several tens of micrometers in diameter, so 300,000x the size of each lithium ion, or a quarter diameter of a typical strand of hair.
Note that lithium ions (blue dots) hanging out in the liquid electrolyte must be escorted (solvated) by solvent molecules (red ovals), typically 3-4 solvent molecules per ion. This is similar to how sodium ions from table salt are surrounded by water molecules in your pasta water. In the case of charging a cell, solvated lithium ions approach the SEI, which then desolvate (a.k.a. say goodbye to their solvent buddies), allowing them to travel through the SEI thickness by themselves, intercalate into the carbon active materials, and finally move toward the center of the active material particle in the solid state. Each one of these steps occurs at its own rate, which means each one contributes a source of internal resistance (fancy term: impedance). You can think of it like going through airport security with TSA:
Mass transport, as we discussed above, refers to how quickly lithium ions can make it through the pores of an electrode to get to the surface of active material particles. It’s like how quickly you can get through the zig-zag line for your turn to strip down and x-ray your stuff.
Charge-transfer resistance refers to how quickly lithium ions can actually desolvate and travel through the SEI layer and intercalate—which they have to do alone. It’s like how quickly you can chug your water bottle, take out your laptop, and feel self conscious about your socks before you make it through the metal detector.
Solid-state diffusion refers to how quickly lithium ions can move within an active material particle once it’s in, and to get the hell out of the way for more coming in. It’s like how quickly you can put your shoes and belt back on so you’re not the bottleneck (stressful), and then get to your gate, dammit.
Each of these sources of impedance are strongly affected by temperature, chemistry, age of the cell, SOC, and more. Which one dominates will also change based upon these factors. Regardless, they need to be properly designed for—on both the anode and the cathode, and they comprise a large part of the polarization in the springs we talked about earlier.
We’re not going to zoom in any further on the chemical composition of the SEI because the honest truth is that nobody knows what it looks like. It’s hypothesized to be a mess of different species, largely dictated by what you put in the electrolyte, which we’ll talk about in the final zoom-in. All we really know for sure is that it’s a solid, it does its job, and we’d be screwed without it.
(Quick aside on lithium plating)
When you can’t put your shoes on quickly enough, or if you keep forgetting to take things out of your pockets, you become the bottleneck and cause the line behind you to backup. In the case of lithiating an anode (a.k.a. charging a cell), this is where some ugly things can start to happen. When solid-state diffusion is too slow or charge-transfer resistance is too high compared to the rate at which lithium ions in the electrolyte are arriving at the anode surface (getting through the zig-zag line), they get pushy and end up depositing onto the anode particle surfaces as metallic lithium. This is known as lithium plating, and it’s bad news.
First of all, SEI immediately forms around metallic lithium—if you thought lithiated carbon had a bad attitude, you obviously haven’t seen lithium. This of course consumes electrolyte and cycle-able lithium, resulting in immediate capacity loss. Second, the additional SEI gunk that grows around the lithium begin clogging the pores of the electrodes, making it harder for lithium ions to make their way through, hurting mass transport and increasing impedance. Finally, as we know from last time, lithium metal doesn’t deposit nicely—it never has. Instead, it forms dendritic structures that can eventually pierce the separator, causing 💥 🔥 . This is the primary reason you cannot charge a Li-ion cell too fast, especially when temperatures are low.
Addressing lithium-plating is therefore a key component to enabling fast-charging batteries for electric vehicles. This is important because typical high-energy density Li-ion batteries can require up to 45-minutes to charge 80%. That number needs to come down to 15-minutes or less to mirror the experience of refueling internal combustion engine (ICE) vehicles. The inability to do so today continues to be a key impediment to EV ownership.
Btw, this phenomenon is also why cathode electrodes are always smaller than anode electrodes. If it were the other way around, the excess amount of cathode around the edges would overwhelm the anode with lithium ions and result in plating around the perimeter, which would be a major safety hazard.
Ok, back from the aside.
2. Cathode-electrolyte Interphase (CEI)
Even less understood than the SEI on the anode is the SEI on the cathode. You can tell because we didn’t even have a name reserved for it until we just replaced “solid” with “cathode” for our CEI. Lazy. We just know there exists an interface which has a significant impact on cycle life, especially in the higher nickel-content cathode materials with higher capacities. Again, the CEI is strongly affected by electrolyte chemistry.
3. Aluminum-electrolyte interface
Remember how we said the components in a Li-ion battery are not necessarily stable and happy? Well that also applies to the aluminum foil on the cathode. It turns out when we charge a cell, the aluminum foil will actually want to corrode at higher voltages (~4V). While some of you may know that a super tough oxide layer (Al2O3; also sapphire) forms on aluminum to give its superb weather-resistant properties out in the rain, that layer is useless inside the corrosive environment of a Li-ion battery.
What actually stabilizes the aluminum is the salt used in the electrolyte. Whereas Li+ is the positive ion (cation) in the salt (similar to Na+ in table salt, NaCl), the negative ion (anion) is usually hexafluorophosphate, or PF6- (similar to Cl-). The salt is therefore LiPF6. It sounds fancy and kinda expensive…both true, but the important part is that it has fluorine, F, in it. This fluorine actually helps form a stable, passivating layer on the aluminum foil surface, made up of AlF3, upon the first charge. And this is what prevents any further corrosion of aluminum1.
Any other salt that does not contain a donate-able fluorine will result in a cell that won’t charge properly, as the aluminum foil will just corrode. Since 1991, we have yet to come up with another salt that’s cheaper or works as well as LiPF6.
Sometimes I get nervous just thinking about how perfectly the stars must align for any battery to even work. Just me? Ok nvm. Let’s continue.
4. Copper-electrolyte Interface
Ok so we can stabilize aluminum, and we like it since it’s light and very conductive. Why can’t we just use the same foil on the anode side of the house? Well…it turns out aluminum will alloy with lithium at the electrochemical potentials experienced in most anodes (we’ll talk about the one exception in the last zoom-in). And typical of any kind of alloying behavior, we will end up with drastic volume changes. Since our foils are only supposed to conduct electrons into and out of cells, we don’t want them doing anything else funky. So, no thanks.
At the very low electrochemical potentials seen by the anode, both copper and nickel are stable. They have similar densities (both are heavy), but since copper is cheaper than nickel, we have our winner.
If a cell is ever overdischarged, though (like going below ~2.0V in a typical ~3.7V cell), the copper foil will also corrode into copper ions and float around in the electrolyte. If you attempt to revive the cell by recharging it because some dude on YouTube swore that it works, the copper ions will deposit as dendritic metallic copper on top of the anode surface, creating a serious risk to short circuiting through the separator and causing 💥 🔥 . Similar to lithium plating. Don’t do it, plz.
And finally…our last zoom
We arrive at our final zoom level where we’ll discuss the crystal and molecular structures of the key materials used in a Li-ion battery. At this zoom level, we find some horrifying pigeon-people on the streets of Japan. Click here if you want to see this on Google Street View yourself. Someone please explain to me what’s going on…
Pigeon-people aside, we’ll be looking into the following components circled below at an even higher resolution than the interfacial-level we just did. Again, we’ll go through them one-by-one:
1. Cathode Crystal Structure
You may recall from the last post that the O.G. cathode material was lithium cobalt oxide, or LCO. Since then, several other cathode materials have been developed, with the usual elemental suspects including nickel, manganese, cobalt, aluminum, and iron. Each one of these materials have unique crystal structures at the atomic level, and they have significant impacts on how they perform. Thankfully, they do fall into three major classes.
Btw, remember from the last post that all of our cathode materials need to be lithiated (containing lithium to start) since the anodes in Li-ion batteries don’t contain any. Cathodes are like the friends that bring the frisbee. See below image of the most prevalent Li-ion cathode classes2:
“Layered” Cathodes (2D): The first and most common class of cathodes, which includes LCO, have the classic layered crystal structure that can reversibly intercalate lithium ions in a shelf-like fashion. Since lithium ions can only move in 2 directions in these shelves, these materials are classified as “2D”, and they were originally discovered by John Goodenough as a structurally stable crystal that can reversibly intercalate lithium ions highly efficiently in the 1980s. Remarkably, this is still the cathode material used in most smartphones and consumer electronics today, but usually not exceeding a certain capacity threshold due to safety reasons. That’s because LCO tends to have poorer thermal stability, despite having good structural stability. Beyond ~150ºC, LCO will begin breaking down exothermically, releasing oxygen trapped inside the crystal which fuels its own fire 🔥 . Fun. Instead of using pure cobalt as the transition metal, people later realized we could blend other metals like nickel and manganese together with cobalt to increase capacity and improve safety. These cathode materials became known as NMC for short, with varying ratios of each component (fancy term: stoichiometry). These ratios have big implications on energy density, safety, and cost, so we’ll do a quick deep dive on them. (For a very interesting backstory on the huge lawsuit behind NMC, I recommend you read this fantastic Quartz piece by Steve LeVine)
The first NMC to be developed contained equal parts Ni, Mn, and Co, hence it was called NMC111 (or NMC333, for ~30% of each), and it increased the thermal stability closer to ~200ºC while maintaining energy density. In general, reducing Co content is desired because it is toxic, expensive, and a “conflict material”—a large part of the raw metal is mined in the Congo with child labor and abysmal conditions. But our cobalt addiction stems from its ability to provide structural stability during cycling, and it’s hard to completely get rid of. Increasing Ni content is generally desired to increase capacity and energy density, but doing so will reduce structural stability, impairing cycle life, while also increasing likelihood of exothermic restructuring while releasing oxygen—again, not good for 🔥 . Increased Ni content also makes these materials ultra-hygroscopic (soaks up water), and exposure to moisture will cause irreversible capacity loss. So these powders need to be kept in super dry conditions from the minute they are manufactured to final sealing inside a Li-ion cell, adding process complexity (just like how lettuce needs to be refrigerated from farm to table with no break in the “cold-chain”…or else E. Coli). Manganese in general acts as a good buffer/stabilizer material that is cheap and non-toxic, but it tends to dissolve into the electrolyte—a process known as “manganese dissolution”—and cause rapid capacity fade. Again, tradeoffs everywhere!
Because we seem to value energy density and low cost above all else, the general trend has been increasing Ni and reducing Co, with Mn filling the balance. So in just the past decade, we have seen the transition from NMC333 —> NMC433 —> NMC532 —> NMC622 —> NMC721 —> NMC811, where 811 represents approximately 80% Ni, 10% Mn, and 10% Co. NMC811 has >40% higher capacity compared to LCO, significantly cheaper due to minimal cobalt content, but roughly the same thermal (in)stability for reasons discussed above. EV battery packs made with it will therefore need to be carefully managed to never exceed temperature thresholds, and significant electrolyte development is required for the thousands of cycles required in cars.
Further increases in Ni content are expected and already being developed, with anticipated ratios >90%, and cobalt contents <3%. However, further reductions to cobalt content for a truly “cobalt-free” cathode would mostly be symbolic and a detriment to cycle life. Though being able to say “cobalt free” turns people on in this industry. In parallel to NMC development, aluminum can also be used as a “dopant” similar to manganese, and these are known as NCA cathodes. Ultimately though, these are non-strict stoichiometries and various dopants are thrown in to stabilize the system. NCA cathodes are the types found in most cylindrical cells, including those used by Tesla.
“Spinel” Cathodes (3D): Whereas layered materials give lithium ions 2 degrees of freedom, cathodes with a “spinel” crystal structure have 3-degrees of freedom. As a result, these structures can have very rapid solid-state diffusion. The only cathode material that has been commercialized with this crystal structure is lithium manganese oxide, or LMO. As we mentioned above, though, manganese is notorious for dissolution into the electrolyte, which will preferentially deposit as dendritic metal onto the anodes (like the copper ions after overdischarge) and result in rapid capacity fade and potential short circuits. It’s always the damn dendrites! Because of this, few batteries today use LMO-only cathodes.
“Olivine” Cathodes (1D): Finally, the third major class of cathode active materials have an “olivine” crystal structure, which only have 1-dimensional tunnels for lithium to travel. And yes, you guessed right that these materials generally have lower rate capability. To combat this issue though, one can just make these active material particles very small (nano-sized) to reduce the total length lithium ions need to travel within a solid. It’s like putting the boarding gates closer to security checks.
The only commercialized olivine cathode material is lithium iron phosphate, or LFP. The great thing about LFP is that it’s non-toxic, made with abundant materials, thermally and structurally stable, and easy-ish to recycle. But of course…there’s always a catch (are you sick of this now?). LFP materials have a double-whammy in that they have lower capacities and lower electrochemical voltages—ultimately hurting energy densities.
This may have been a death-spell for LFP just a couple years ago for the EV market, but given the demand for low-cost, mass-market EVs that don’t necessarily need to drive you cross-country, LFP is making a remarkable comeback. They are now being used for the lower-end versions of electric vehicle lineups. Plus, they have that extra “cobalt-free” flex that everyone loves to taunt.
Additionally, since LFP cathodes are significantly safer than other chemistries, manufacturers have realized they can start making gigantic cells—much larger than any sane person would design for NMC-based chemistries—to help with reducing packaging overhead. Whereas conventional EV batteries are made up from individual cells packaged into modules, which are then strung together in rows, and finally combined into packs, ginormous LFP cells can now be directly stitched together to form much simpler pack designs. This strategy is known as “cell-to-pack”, or C2P. This certainly helps with LFP’s energy density handicap, but doing the math will tell you that it will never be enough to out-energy-dense (new phrase) high-nickel cathode chemistries like NMC811.
2. Anode Crystal Structure
After 30 years of Li-ion commercialization, we have still not yet graduated from the carbon host anode materials originally proposed by Yoshino. We’ll understand why by looking at some of the key anode material candidates shown below3. Again, note that the images below show the crystal structures of anodes after being lithiated (in a charged state), because they don’t contain any usable lithium inherently—anodes are free-loaders.
Graphite (carbon) Anodes: The first Li-ion battery commercialized used petroleum coke as the carbon host material, and it eventually got replaced with graphite, which is still the dominant anode material used today. Graphitic materials are layered sheet-like structures of individual graphene planes of a never-ending hexagonal pattern of carbons. The degree to which the entire material comprises this neatly stacked structure is known as the degree of crystallinity or graphitization, and graphite materials have, you guessed it, high degrees of it. The way in which graphite hosts lithium ions is to keep them in between the sheets 😏 , not unlike the 2D layered cathode materials we met earlier. Each ring of six carbon atoms can host a maximum of one lithium ion.
There are many types of graphite materials but they can all be traced back to two raw material origins—naturally mined as flakes or synthetically graphitized from petroleum feedstocks. The synthetic (a.k.a. artificial) graphitization process requires very high temperatures and is therefore an energy-intensive process that can be considered “dirty”, whereas natural graphite requires purification with nasty stuff like sulfuric acid. However, the beauty of graphite is that lithium intercalation is highly reversible for long cycle life, it experiences relatively low volume expansion for good mechanical stability, its electrochemical potential is close to pure lithium that helps maximize energy densities, and it has a decently high capacity.
For some time now, the next major breakthrough in lithium-based batteries is almost always seeking to displace graphite as king. Attempts continue to be made with silicon anodes (which we’ll talk about next) and pure lithium metal (which we’ll talk about next time), but so far, nothing has come close to being able to displace it completely. Despite its flaws, graphite still remains a remarkable anode material.
Silicon-based Anodes: Whereas six carbon atoms can host one lithium ion, one silicon atom can bond with four lithium ions. It ain’t hard to do the math that silicon has a significant advantage in capacity for lithium storage. This is the sexy part many people have been chasing for a while now…but with little success. Unlike graphite or the cathode materials we met earlier, silicon is not an intercalation material that hosts a guest lithium species. Instead, it gets nice and intimate with lithium since it is a conversion material that reversibly forms a lithium alloy. And just like we learned earlier with aluminum foil, alloying stuff results in tremendous (not in a good way) volumetric expansion. So while silicon has more than 10x the capacity of graphite on a per gram basis, it will also expand by ~300%. This volumetric expansion is the root of most problems with silicon—a simple one that anyone can understand, but actually turns out to be one of the hardest problems to solve. Here is a decent graphic showing what happens to silicon4:
With such dramatic volume changes, the SEI blankets that we discussed earlier naturally get ripped open with each charge, revealing more surface area that must form new SEI. This is kinda like the Hulk needing to buy new clothes every time he gets big, green, and angry. Not very sustainable. After many cycles, the silicon particles end up pulverizing themselves into smaller and smaller pieces, which again reveal new surface area that must be blanketed. Every time new SEI is formed, lithium is irreversibly consumed, causing rapid capacity fade. Additionally, pulverized silicon active materials can get dislodged from their CBD network and become electrically isolated. Without a way for electrons to access the particles, no lithium can get in and out, and so electrically isolated silicon lose their ability to contribute capacity. All of this mess just means that cycle life with silicon sucks.
Still, the promise of 10x the capacity of graphite (which does not translate to 10x the energy density) is meaningful enough that we continue to try to find workarounds. Some key strategies include making silicon particles super tiny to better resist pulverization, using intermediate compounds like silicon oxides as a halfway point, and locking silicon particles in carbon cages so they can’t wreak as much havoc. The dream used to be using pure silicon as an anode, but now the goal has evolved to “silicon-dominant” anodes, with good ol’ graphite filling the rest. Again, it’s hard to displace graphite completely.
Today, most commercial Li-ion batteries already incorporate small amounts of silicon-based active materials in a predominantly graphite anode—typically no more than 7-10%. This provides a decent boost to energy densities without dramatically impairing cycle life, but cycle life is certainly compromised to some degree.
Lithium Titanate Anodes (LTO): Our final class of anodes is one that has always been on the fringes for niche applications, and will likely never escape that predicament. LTO anodes, which can sometimes be confused as a cathode material since it has an “L” and “O” in it, is a crystal that also has a spinel structure—similar to our LMO example above. But unlike cathode materials, LTO actually works by absorbing more lithium ions, thereby functioning as an anode. Like LMO, the 3D-structure means it has significantly higher rate compared to the 2D-structure of graphite, so LTO anodes can lithiate (charge) and delithiate (discharge) very rapidly. But they suffer from a similar double-whammy fate as LFP in that they have low capacity (roughly half of graphite), and their electrochemical potential is higher. Since the voltage of any Li-ion battery is the difference between the cathode voltage (always higher) and the anode voltage (always lower), a higher anode potential results in a lower overall full cell voltage. As a result, LTO cells have significantly lower energy densities, and will see a quadruple-whammy if paired with LFP cathodes—being quite the impractical pairing.
The high electrochemical potential of LTO anodes has two interesting things going for it, though. (1) LTO does not really form an SEI layer since it’s less grumpy and reactive with the electrolyte at high voltages, and (2) aluminum foil can be used as the current collector for LTO since lithium will not alloy with aluminum at those potentials. This helps reduce cell weight, but not nearly enough to make up for the other energy density penalties. Because of this, LTO chemistries are relegated to only niche applications that need extremely high charge/discharge rates.
3. Electrolyte Composition
Our final component is the electrolyte, and it’s fitting because the electrolyte is the last thing to be injected into a cell and also the component that must pick up after everything else. Last time we said that conventional batteries are like the moms of electrochemical storage systems. Well, electrolytes are like the moms of Li-ion batteries. They need to tolerate the high voltage window inside a Li-ion cell, put up with the anode and cathode’s B.S., make sure everyone is communicating with each other, and they’re the only thing that touches every component 🥺 ❤️ . Just like we had our list of demands for electrochemical energy storage last time, here is our list of demands that we need our electrolytes to fulfill:
Again, it’s remarkable we already have working systems that give you thousands of cycles in an environment like a Li-ion battery. But because of the need to satisfy so many demands, electrolyte formulations end up being a jumbled mess of different components, each designed to help with one demand or another. As always, helping in one area most likely hurts another, so it’s yet again a game of tradeoffs.
Typical electrolyte formulations have the following required and optional components:
Lithium Salt-bae (required): The thing that is dissolved by the electrolyte solvent (like salt in water) that provides lithium ions in the electrolyte solution. Adding more salt doesn’t increase your cell capacity because the lithium in salt exists as positive ions (cations) only and don’t have electrons to go along with them. Only lithium stored in the active materials have electrons to contribute electricity, so capacity is limited to only those amounts. The negative ions (anions) from the salt generally float around aimlessly, but are occasionally enlisted to help form a passivating layer on the aluminum foil. Increasing the concentration of salts can increase conductivity up until a certain point, after which it falls (and then rise again at ultra-high concentrations). But increasing concentration also increases viscosity, and we don’t like goopy things.
Solvent (required): These molecules are required to solvate and dissociate the cations (Li+) from the anions. They need to be liquid so they permeate all components and fill up the pores. They are also likely to be key building blocks in the formation of SEI on the anode and CEI on the cathode. Solvents are typically a blend of different organic, non-aqueous carbonate-based molecules.
Additives (optional, but you’d be foolish not to use them): Additives are anything that is added in trace amounts (e.g. <5%) to assist in addressing some of the demands listed above. Just a soupçon. They can be salts and other solvent molecules to improve SEI quality, diluents for increased wettability, or other fancy things to assist elsewhere (cathode stability, low-temperature performance, fast-charge, safety). Since additives are used in such low quantities and often chemically react irreversibly upon the first charge/discharge cycle that manufacturers do in-house, they are very hard to reverse-engineer. The additives used in commercial cells are therefore highly secretive recipes.
Co-solvents (optional): Additional solvent species that help reduce viscosity of the electrolyte for improved power performance or improved wetting behavior. These can be counterproductive for safety purposes though, as they can increase volatility.
All zoomed out!
And there we have it. If you made it this far, you now have a decent foundational understanding of Li-ion batteries from the ground up, starting at the pigeon-people level. Through our zoom-in process, we navigated 9 orders-of-magnitude in length scales from centimeters to Ångstroms (1/10th of a nanometer). If we travelled the same span to end up at our pigeon-people friends, we’d actually have to start our zoom-in process from 1.6 million kilometers from Earth, which happens to be where NASA’s DSCOVR satellite sits at the Lagrange Point 1—a gravity neutral place in space. This is what that actual view of Earth looks like from that far5:
What a precious lil’ planet we live on. All the more reason we need to get our act together and transition to clean energy, with Li-ion batteries playing a key role in that process. Again, we’ll discuss Li-ion’s role at the end of the next post.
Now that we’ve understood how Li-ion batteries are supposed to work, you are now fully equipped (or close to it) to understand this lovely graphic showing all the different ugly failure mechanisms a Li-ion battery can experience6. When battery engineers have nightmares, this is what they dream about:
What is especially useful from the above graphic is understanding that batteries are hard. The Li-ion cell is an engineering marvel because we have managed to first understand and then address (to a reasonable degree) each of the above failure mechanisms—sometimes by ingenuity, sometimes by brute force, and sometimes by luck. But now that you are grounded in the realities of battery engineering, “battery breakthroughs” and “h*ly gr*ils” should come to you with great skepticism. These are complex systems with few working solutions and tradeoffs aplenty.
Armed with all this nerd power, we are now also equipped to deep dive into lithium-metal and solid-state batteries in the next post. Spoiler alert: Don’t hold your breath for either of them to power your car anytime soon…we’ve still got a ways to go.
Despite their shortcomings, Li-ion batteries are undoubtedly here to stay for the long haul, and they are likely to remain the incumbent technology—especially for electric vehicles—for at least the next ten to fifteen years. I’d celebrate if I were wrong, but that’s the more likely and inconvenient truth. Sorry if I burst a bubble.
TL;DR
We did 6 levels of zooming in and traversed 9 orders-of-magnitude in length scales
Li-ion cells are an engineering marvel, and there seem to be more ways for things to go wrong than right
Batteries are hard. We’ll talk about lithium-metal and solid-state batteries next time, but they won’t be powering your car anytime soon
Thanks for sticking with me to the end, and I look forward to continuing our jam-sesh in the next deep dive. If you want to show some big pic energy, please share with your nerd friends to subscribe:)
Thanks and much love,
Adrian
Zhu, P., Gastol, D., Marshall, J., Sommerville, R., Goodship, V., & Kendrick, E. (2021). A review of current collectors for lithium-ion batteries. Journal of Power Sources, 485, 229321. https://doi.org/10.1016/j.jpowsour.2020.229321
Julien, C., Mauger, A., Zaghib, K., & Groult, H. (2014). Comparative Issues of Cathode Materials for Li-Ion Batteries. Inorganics, 2(1), 132–154. https://doi.org/10.3390/inorganics2010132
Okoro, F. (2018). Li-ion Batteries for Electric Mobility. Unpublished. https://doi.org/10.13140/RG.2.2.36748.77446
Choi, J. W., & Aurbach, D. (2016). Promise and reality of post-lithium-ion batteries with high energy densities. Nature Reviews Materials, 1(4). https://doi.org/10.1038/natrevmats.2016.13
https://www.nesdis.noaa.gov/content/dscovr-deep-space-climate-observatory
Birkl, C. R., Roberts, M. R., McTurk, E., Bruce, P. G., & Howey, D. A. (2017). Degradation diagnostics for lithium ion cells. Journal of Power Sources, 341, 373–386. https://doi.org/10.1016/j.jpowsour.2016.12.011